Expanding the Genetic Code: Sense Codon Reassignment
One of the driving motivations of both chemical and synthetic biology is the expansion of the set of building blocks that can be employed in the templated synthesis of biopolymers. The expansion of the set of amino acids that can be utilized in translation is particularly challenging. Genetic code expansion is hampered by the fact that all 64 codon triplets have an assigned function. However, the genetic code is degenerate: 61 sense codons specify 20 canonical amino acids.
Expanding the genetic code by reassigning the meaning of sense codons should be broadly generalizable. Unfortunately, predicting which sense codons are amenable to reassignment and which orthogonal machinery is best suited for the task is made challenging by the largely unknown and idiosyncratic recognition and discrimination features of each organism’s complement of tRNAs and aminoacyl tRNA synthetases (aaRSs).
We are opening new codons to reassignment by breaking the degeneracy of the genetic code. Further, we integrating sense codon reassignment technology with the improved technologies for non-canonical amino acid incorporation in response to amber stop codons to generate 22 and 23 amino acid codes where multiple copies of multiple non-canonical amino acids can be incorporated into proteins of interest. The improved technology along with presently available suite of non-canonical amino acid incorporating enzymes expands the number of available genetic codes from 100’s to 10,000’s to 1,000,000’s (from 21 (~200) to 22 (~100)2 to 23 (~100)3 codes) opening vast new vistas of protein sequence and functional space for exploration and exploitation. Breaking the degeneracy of the genetic code by reassigning the meaning of sense codons provides an additional avenue through which biosynthetic modifications can be made, furthering both fundamental and applied biochemical research.
The Fisk Lab's fluorescence-based screen for sense codon reassignment. Our screen evaluates the ability of anticodon-modified tRNAs to incorporate tyrosine (Tyr) in response to a sense codon that is assigned another identity in the standard E. coli genetic code. Residues 65-67 of superfolder GFP specify the Thr-Tyr-Gly sequence that autocatalytically folds into the tripeptide fluorophore. Replacement of Tyr at position 66 with any other natural amino acid effectively abolishes the fluorescence of the protein. (Biochemistry, 2015, 54 (50), pp 7355–7364)
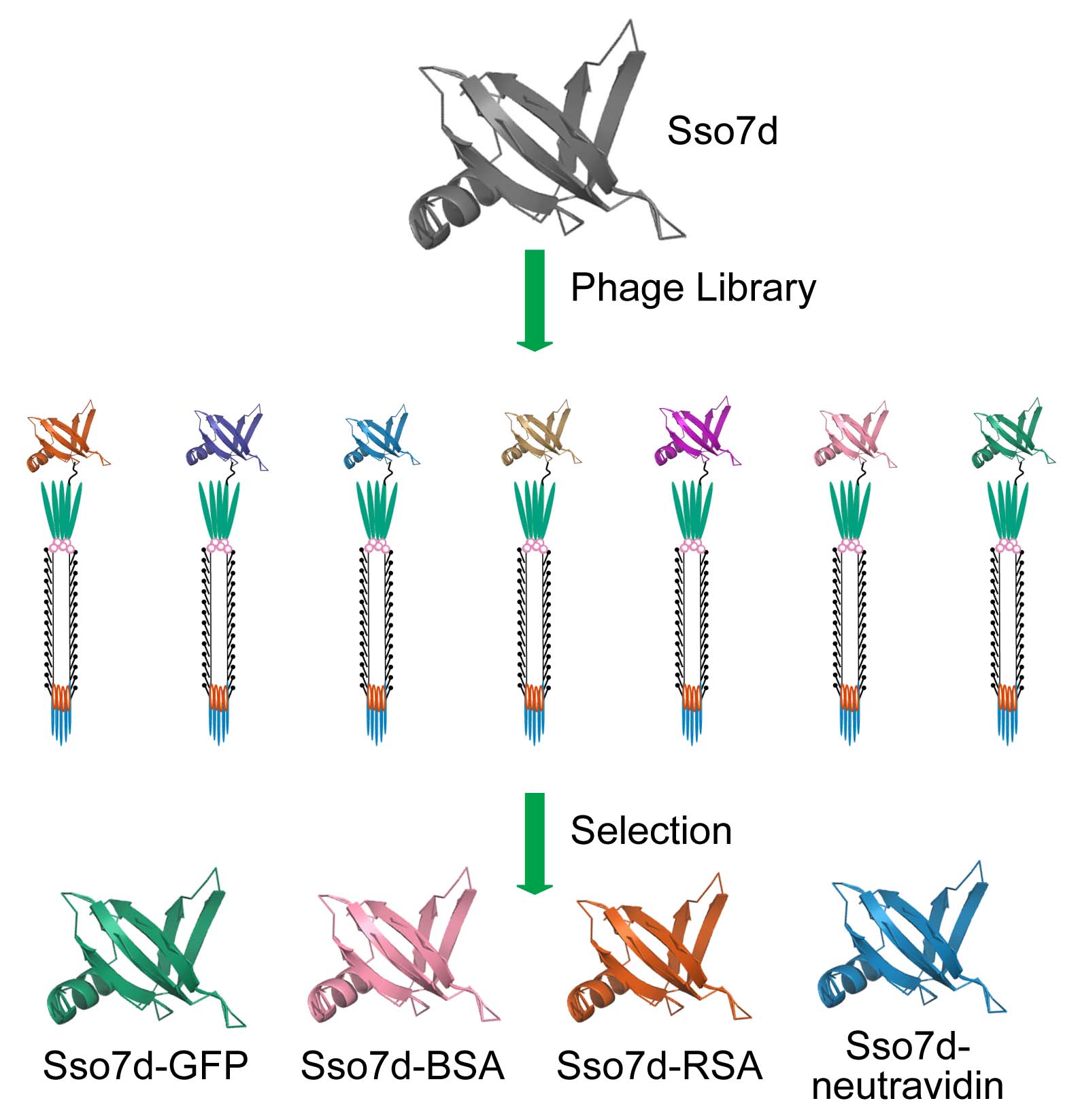
Phage Display and Phage-based Materials
We are exploring the development of highly modified bacteriophage particles as replacements for traditional antibody reagents. These new components derive their improved characteristics by combining the presentation of biological recognition and signal detection moieties as appendages on bacterial virus particles. The ability to display multiple binding and signaling molecules on a particle allows for improved behavior in two ways: increased tunability of response (by controlling the number of available binding molecules) and improved sensitivity through avidity effects. In a similar way, the ability to display multiple signal generating components on a particle can lead to a more robust response. Signal amplification can occur through the attachment of multiple fluorescent or catalytic domains to the particles: one binding event can immobilize 10’s to 100’s of fluorescent molecules or enzymes. Because all of the components of the sensors are created and assembled as part of the phage life cycle process, these sensors will be incredibly inexpensive to manufacture. Phage particles have been shown to increase the stability of proteins appended to them, and this effect may the extend shelf life of phage-based sensor components. We are working to develop these types of reagents as components of diagnostics ideally suited to the challenges and demands of resource-poor areas. However, the low cost, ease of use and long shelf life of phage-based sensors could be transformative in all health care settings.
The Fisk lab is interested in identifying new scaffold proteins for phage display libraries. We have had success identifying tight, specific binding molecules for a variety of protein targets from a library based on the 65 amino acid hyperthermophilic scaffold protein Sso7d. (FEBS J, 2016, 283, pp 1351–1367)
Computational Simulation of the M13 Life Cycle
Where does one begin when trying to build a complex biotechnological system with a defined function? Many of the basic genetic components of biotechnological infrastructure (e.g. DNA plasmid constructs, promoters and ribosome binding sites) available today are the result of a random walk of incremental changes to parts co-opted from myriad organisms rather than a careful, considered design of systems with particular function that operate in clearly understood ways. While molecular biology and bioengineering have flourished by combining these parts, the next great leap of synthetic biology and bioengineering will be the ability to impose an engineering design cycle on biological structure and function. Research in the Fisk lab aims to contribute to this next step at both the fundamental and applied levels. This approach must begin by understanding the complexities of Nature, specifically by forming a quantitative description of biological interactions. Quantitative biology enables the construction of quantitative models that allow for the directed engineering of new useful functions into biological systems.
As the biotechnological applications of M13 phage particles continue to expand in scope and complexity, a quantitative, holistic understanding of the biological processes and interactions that govern the life cycle of the phage will foster the creation of new platforms with rationally designed control elements that are more amenable to engineering. We were inspired by work from Drew Endy and Lingchong You in John Yin’s laboratory that described the biology of the lytic T7 bacteriophage quantitatively via a chemical kinetic simulation. They noted, and we also believe, that understanding biology at a level that includes all of the biological components in a system and considers the timing of their production, interaction and function, is a necessary starting point for real, model-based engineering of biological systems.
We have constructed a genetically-structured, experimentally-based computational simulation of the life cycle of the non-lytic filamentous bacteriophage M13 to evaluate and expand the system level understanding of this biotechnologically-relevant virus. Our deterministic chemical kinetic simulation integrates 50 years of experimental observations and explicitly includes the molecular details of phage DNA replication, mRNA transcription, protein translation and phage particle assembly, as well as the competing protein-protein and protein-nucleic acid interactions that control the timing and extent of phage production. Many aspects of M13 biology are faithfully reproduced by the simulation, including the production levels and timing of the shift between synthesis of replicative form and preassembly complex DNA, quantities of phage mRNA and proteins, and the time course of assembly and release of progeny phage. The simulation serves as an important step in the process of developing a quantitative understanding of the complete set of interactions controlling M13 biology. The simulation provides a platform for investigating complex interactions between phage components and is a tool to predict points for productive redesign.
The Fisk lab is interested in exploiting the self-replicating, self-assembling M13 phage particle as a platform for multivalent, mulifunctional biotechnologies. This goal requires a complete understanding of the quantitative biology involved in the entire M13 life cycle. Our first two papers developing and utilizing a chemical kinetic simulation of the entire M13 life cycle have been published. (Virology, 2016, http://dx.doi.org/10.1016/j.virol.2016.08.017 and http://dx/doi.org/10.1016/j.virol.2016.08.015)